pH Measurment made
Simple.
Fun.
EZ.
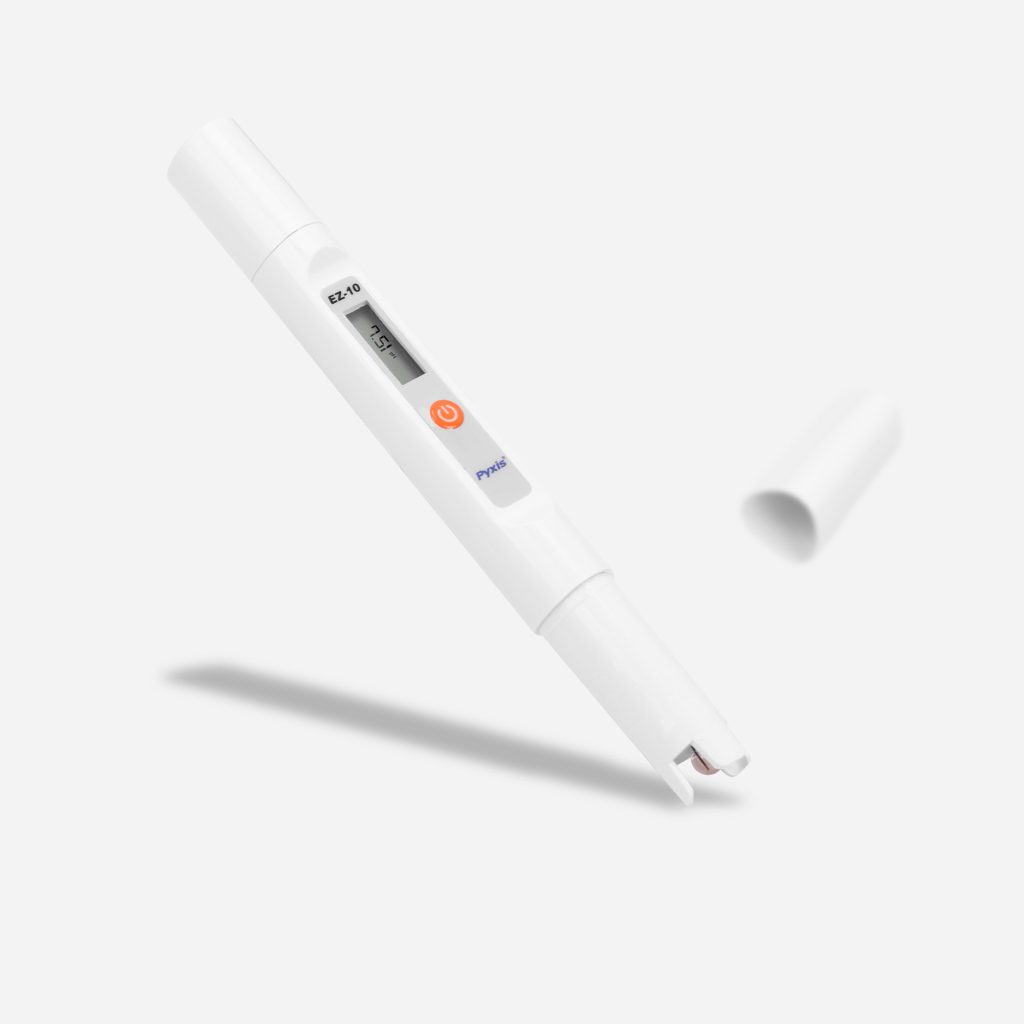
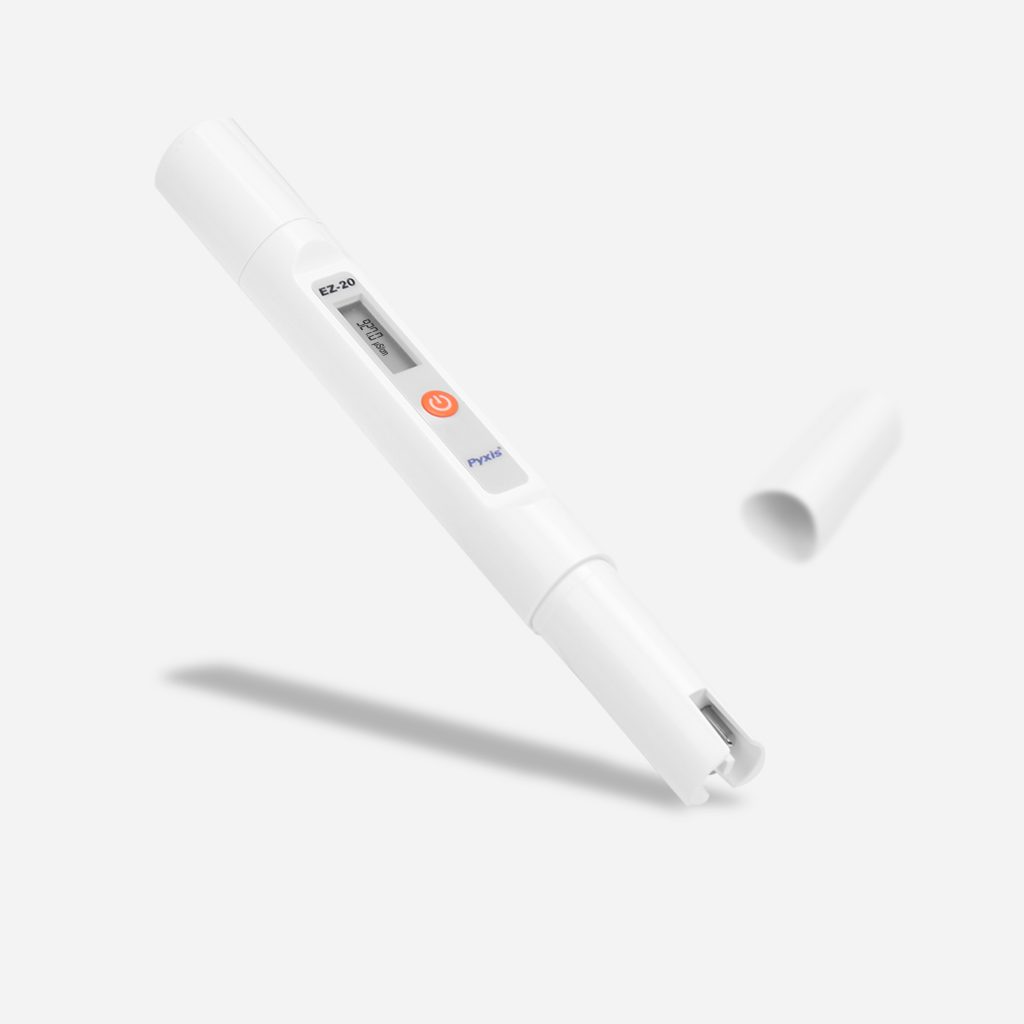
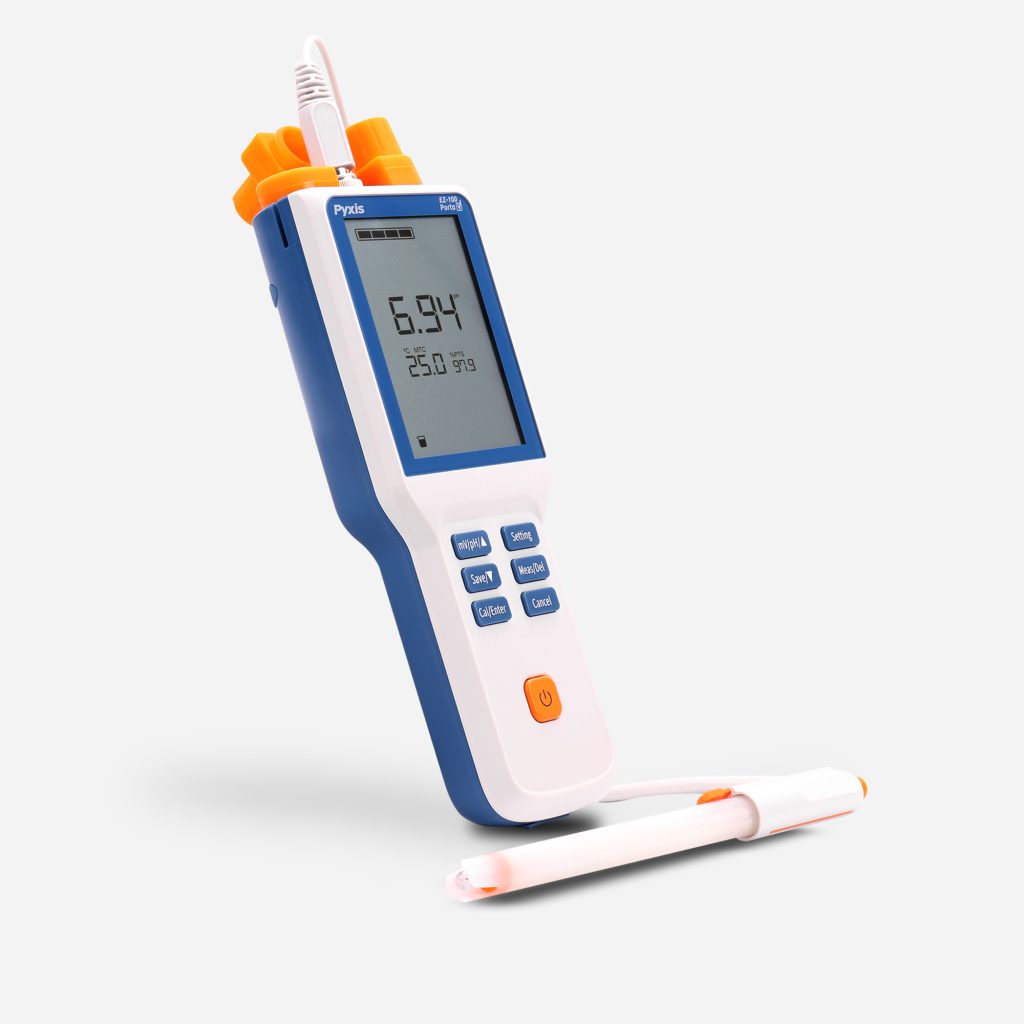
EZ-Series of pH & Conductivity Meters
Introducing an all-new, simplistic series from Pyxis Lab® of pH and Conductivity meters built for the lab, classroom or the water treatment facility. Measure for pH (0–14) and Conductivity (0–2,000µS/cm) as well as temperature. At Pyxis Lab we create extremely innovative sensors and devices for key water treatment parameters, so this series came naturally & EZ to us!
What is pH & Why is it Important in Water Treatment?
pH is a measure of the acidity or alkalinity of a solution. It is defined as the negative logarithm of the concentration of hydrogen ions in the solution. The pH scale typically ranges from 0 to 14, where a pH of 7 is considered neutral. Solutions with a pH less than 7 are acidic, while solutions with a pH greater than 7 are alkaline (or basic). The pH scale is logarithmic, meaning each whole pH value below 7 is 10 times more acidic than the next higher value, and each whole pH value above 7 is 10 times more alkaline than the next lower value.
pH plays a crucial role in water treatment applications for several reasons:
Chemical Reactions: The pH of water affects the chemical reactions that occur during the treatment process. For example, the effectiveness of disinfection processes such as chlorination can be influenced by pH.
Corrosion Control: pH can impact the corrosiveness of water. Water that is too acidic or too alkaline can corrode pipes and equipment, leading to damage and potentially harmful leaks.
Coagulation and Flocculation: In processes like coagulation and flocculation, where particles are clumped together for easier removal, the pH of the water can affect the efficiency of these processes.
Ionization of Chemicals: The pH of water can affect the ionization of chemicals used in treatment processes. For example, in ion exchange processes, the pH can impact the removal of specific ions from the water.
Biological Processes: pH can also affect biological processes in water treatment, such as the activity of microorganisms used in wastewater treatment plants.
Overall, controlling and monitoring pH is important to ensure the efficiency of water treatment processes and to maintain the quality of treated water.